Could the supplement industry do better? Whether you’re a consumer, a healthcare practitioner, an investor, or part of a company working in the field of wellness, there’s a good chance that this industry affects you in some way—and so do the challenges the supplement marketplace will be facing in the near future.
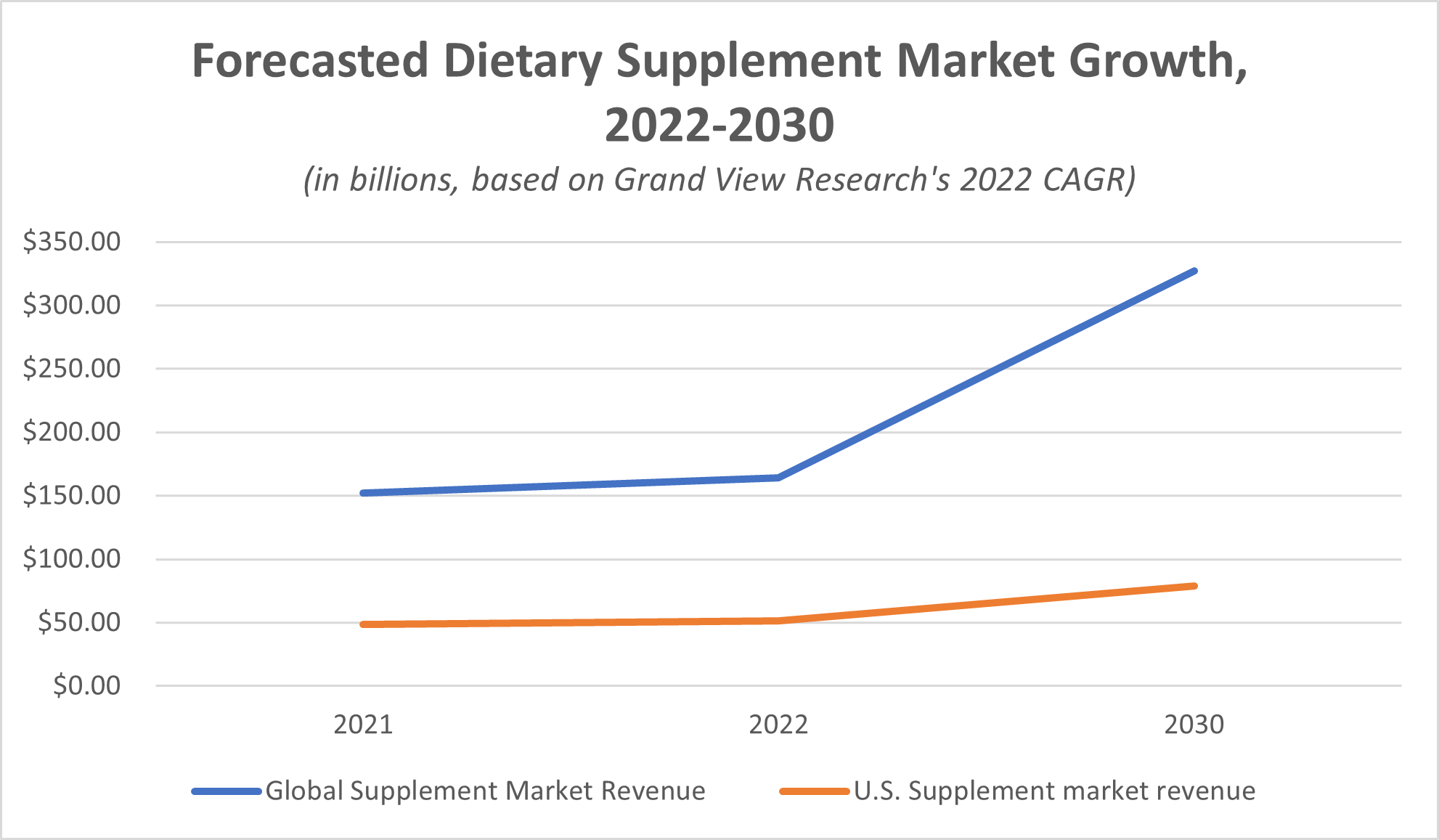
The supplement industry has experienced such extensive market growth that market research and consulting firm Grand View Research anticipates global revenues to nearly double between 2021 and 2030. Despite the undeniable success of the market, this industry isn’t perfect. Some of the biggest issues in the supplement marketplace include contamination, the potential for health risks, and concerns over regulation (or lack thereof).
Dietary Supplement Basics
Dietary supplements are, as defined by the Dietary Supplement Health and Education Act of 1994 (DSHEA), non-tobacco products that include one or more dietary substances or ingredients as listed and are intended to be ingested in approved forms as a supplement rather than conventional foods.
Generally, supplements offer health and wellness benefits of some kind, but they aren’t medicines. What distinguishes supplements from medications like prescription or over-the-counter drugs is that, according to the National Institutes of Health Office of Dietary Supplements, supplements are only meant to supplement, enhance, or complete a consumer’s diet. They aren’t meant to prevent, diagnose, treat, mitigate, or cure diseases or health conditions, the NIH Office of Dietary Supplements reported.
As such, you’re likely to find statements on the labels of dietary supplements that contain language such as “This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease.” However, you may find other types of claims on supplement labels, including health claims that indicate the relationship between the supplement’s ingredients and a decrease in disease risk, according to the NIH Health Office of Dietary Supplements.
According to the National Institutes of Health Office of Dietary Supplements, the ingredients that make up dietary supplements often include vitamins, minerals, amino acids, enzymes, and herbs and botanical ingredients. Dietary supplements come in many forms, from capsules, tablets and powders to gummies, energy bars, and drinks.
Increasing consumer awareness of the importance of health and wellness has driven significant increases in the use of dietary supplements for a variety of purposes. Pregnant women take prenatal vitamins. Supplements that include ingredients like vitamin C and zinc may claim to prevent or shorten the duration of the common cold. Among American adults in all age groups (as well as children), daily multivitamin-mineral supplements are popular. Athletes and exercise enthusiasts often take sports nutrition supplements, like protein supplements, pre-workout powder supplements, amino acids, weight loss supplements, and creatine, to boost their workout performance and the results they get from exercise.
Just how popular are supplements? As of 2022, three-quarters of American adults surveyed reported using dietary supplement products, according to the Council for Responsible Nutrition Consumer Survey on Dietary Supplements.
Contamination in the Dietary Supplement Industry
Adulteration or contamination is a major area of concern in the dietary supplements market.
Types of Contamination in the Dietary Supplement Industry
The American Medical Association (AMA) Journal of Ethics identifies two main types of adulteration found in dietary supplements. Economic adulteration involves swapping a less expensive ingredient for the more expensive ingredient listed on the label. Pharmaceutical adulteration occurs when the product includes an active ingredient that doesn’t appear on the label.
Adulteration of dietary supplements that includes contamination with unlisted ingredients is an alarmingly common issue. The unlisted ingredient may be a drug that is approved by the FDA but not authorized for use in the dietary supplement or a drug that is only approved in other countries—or, potentially, an experimental drug not approved for human consumption anywhere, the AMA Journal of Ethics reported.
The Dangers of Contaminated Supplements
In many cases, what is found to contaminate a dietary supplement is a substance banned by the World Anti-Doping Agency or by individual sports leagues and associations (professional or otherwise). Athletically banned substances include anabolic steroids that, while they may be effective at enhancing sports performance, are not legal or safe and may, according to Mayo Clinic, pose serious health risks. Additionally, athletes and competitors who are caught using banned substances—even unknowingly due to the consumption of adulterated supplements—may be disqualified from competing further.
Accusations of cheating are far from the only risk associated with adulterated dietary supplements. Adverse events from taking adulterated dietary supplements give rise to an estimated 23,000 visits to the emergency room annually, the American Medical Association Journal of Ethics reported. Certain dietary supplements are more commonly contaminated than others. Among the types of dietary supplements in which pharmaceutical adulteration is most prevalent are sports supplements, weight loss supplements, and supplements for sexual enhancement, according to the AMA Journal of Ethics.
Can Dietary Supplements Be Dangerous?
Dietary supplements can pose a risk of side effects, adverse events, and other health issues.
Whether because consumers tend to view them as “just” dietary ingredients or because of a tendency to equate “natural” to “safe” when it comes to a botanical ingredient or naturally occurring amino acids, there’s a widespread belief that consuming supplements is unlikely to cause any harm.
While it’s true that the likely outcomes of a supplement that doesn’t deliver on the intended health benefits are wasted money and a relatively harmless excess of nutrients, taking too much of even extensively studied vitamins can put one’s health at risk. Supplements, in general, affect the body, and negative effects are possible, especially when consumed in excess or by someone who is also taking pharmaceutical medications, the American Medical Association Journal of Ethics reported. Among the most serious adverse events that have been reported to arise from the consumption of dietary supplements are heart problems, kidney problems, and even cancer.
Take vitamin E, for example. In conducting a large, years-long clinical trial by the National Cancer Institute, part of the National Institutes of Health (NIH), researchers explored the notion that taking vitamin E dietary supplements might have the potential to prevent prostate cancer. Ultimately, the researchers behind the SELECT trial concluded that the human subjects in the trial who took a vitamin E dietary supplement on its own (without selenium, another supplement that was being tested for its potential in prostate cancer treatment) actually saw their prostate cancer risk increase by 17%, rather than decreasing.
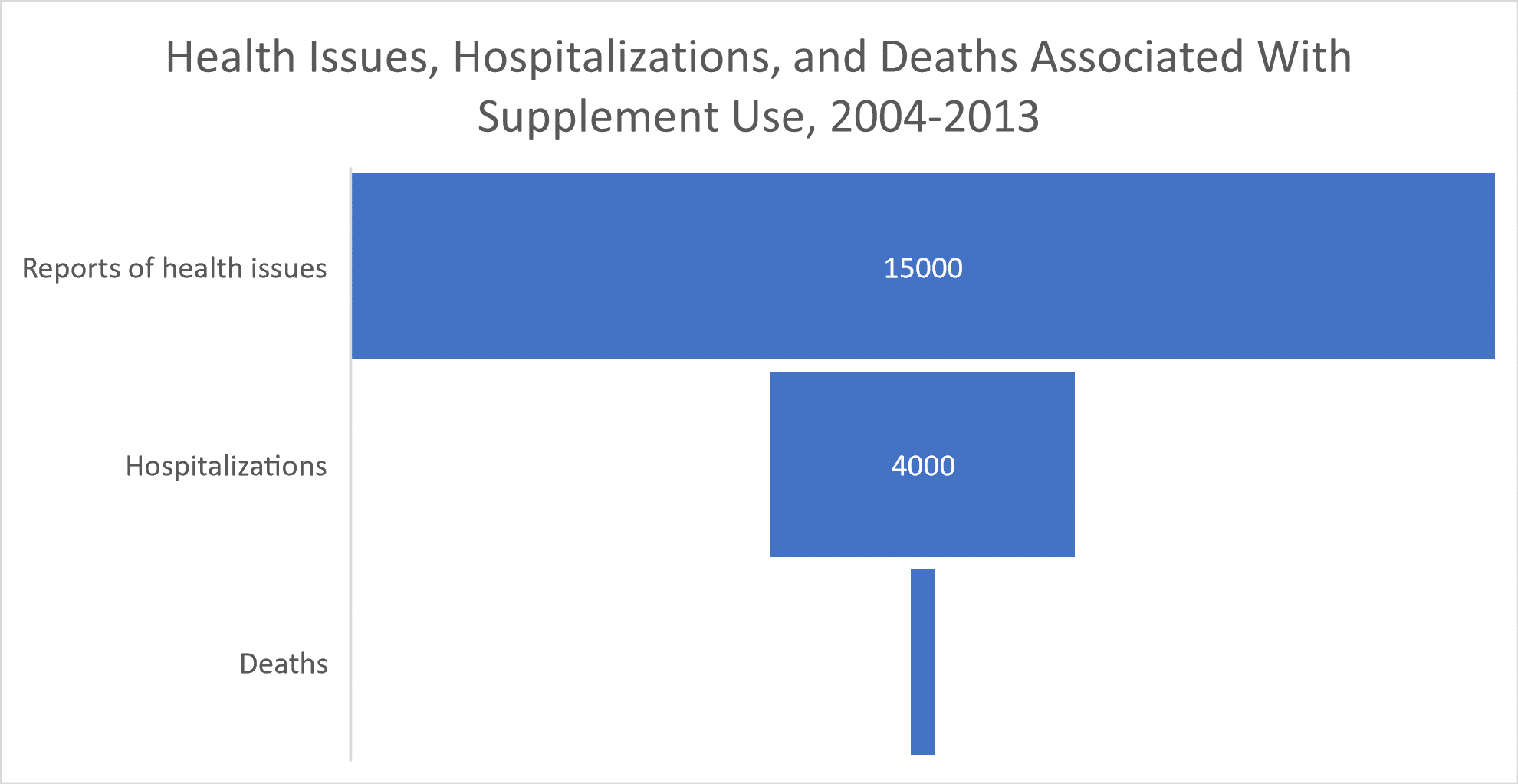
How common are side effects and adverse events with supplement usage? According to The Pew Charitable Trusts, upwards of 15,000 health issues linked to dietary supplements were reported to the FDA between 2004 and 2013, and one out of eight adults survey reported either personally suffering a “severe side effect” from taking a supplement or having an immediate family member who did.
Regulation of the Supplement Industry
Precisely because of matters like contamination and potential health risks, there have been widespread calls for further regulation of the dietary supplement industry. Stricter regulations on the approval and marketing of dietary supplements could help protect consumers from adulterated supplements and ingredients that have a greater potential to lead to serious adverse events.
How the Supplement Industry Is Regulated Today
The regulation of dietary supplements in the United States was established under the Dietary Supplement Health and Education Act of 1994. Although dietary supplements constitute neither conventional foods nor pharmaceutical medications, the U.S. Food and Drug Administration is the federal regulatory agency responsible for overseeing dietary supplements of all kinds.
Many consumers believe that dietary supplements, like pharmaceutical products, are thoroughly tested before they make it to the market and must be approved—and found to be safe and effective—by the FDA before they are sold in America. That’s not true.
Legal website J.D. Supra reported in January 2022 that the legal requirements for putting a new dietary ingredient supplement on the market can be as easy as filing a new dietary ingredient notification (NDIN). Unlike a prescription drug, dietary supplements aren’t required to be shown to be safe and effective through a series of clinical human trials.
What Does the Dietary Supplement Health and Education Act of 1994 Establish?
The federal law that concerns dietary supplements, the Dietary Supplement Health and Education Act of 1994, established a definition of dietary supplements and situated it within the category of foods rather than drugs. This law also established the approval process for new dietary supplements and specified that dietary supplements should be manufactured in accordance with Good Manufacturing Practices in an effort to ensure quality and safety.
Under the official DSHEA wording, the Dietary Supplement Health and Education Act of 1994, the burden of proving that dietary supplements are adulterated, contaminated, or otherwise unsafe falls on the Food and Drug Administration. This is a major departure from the regulation of the pharmaceutical industry, in which manufacturers must prove a drug’s safety and efficacy before it makes it to the market. The enforcement actions the FDA is authorized to take include removing “unsafe” supplements from the market, but there are limitations.
What’s missing from the Dietary Supplement Health and Education Act of 1994 is the authority that would allow the Food and Drug Administration to act quickly to take enforcement actions, protect consumers, and ensure patient safety. Because the regulatory agencies are only authorized under the Dietary Supplement Health and Education Act of 1994 to remove supplement products under certain conditions and after meeting the burden of proof, the FDA can’t take preemptive action—or, in many cases, even act quickly in matters of public health.
The FDA’s Dietary Supplement Ingredient Advisory List
The FDA does maintain a Dietary Supplement Ingredient Advisory List of ingredients that, it said, “do not appear to be lawful ingredients in dietary supplements.” As of February 2023, this list includes the stimulants 1,4-DMAA and N-methyltyramine, the investigational selective androgen receptor modulator andarine, the salt bismuth nitrate, the gold salt sodium tetrachloroaurate, and the synthetic chemical sulbutiamine.
Being on the FDA’s Dietary Supplement Ingredient Advisory List simply means that the regulatory agency is evaluating an ingredient, not that the ingredient has been officially pulled from the market. The FDA has clarified that it can only remove dietary supplements from the market if it can “first establish that such products are adulterated (e.g., that the product is unsafe) or misbranded (e.g., that the labeling is false or misleading).”
As such, just because dietary supplements have been put on the market doesn’t mean they have been tested for safety and efficacy in the same way that pharmaceutical medications have.
Comparing Regulations in the Supplement Industry to the Pharmaceutical Industry
Let’s compare the regulation and oversight of the dietary supplements industry with that of the pharmaceutical drug industry. Both industries produce products that are consumed for health-related reasons and officially answer to the same regulatory agencies, but only one is highly regulated.
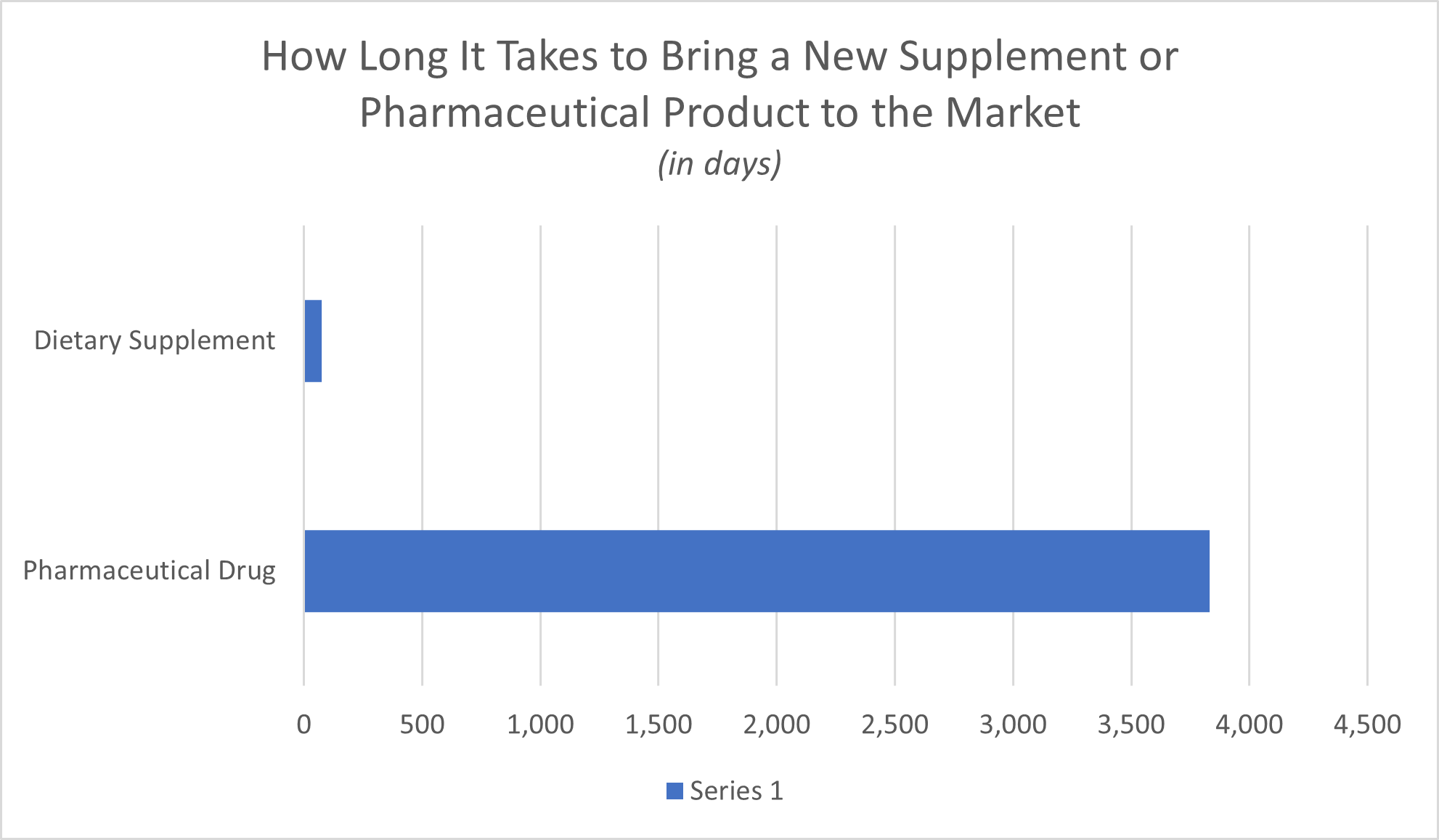
Under FDA regulations, pharmaceutical drugs must undergo a multi-step drug development process that includes numerous phases of clinical testing conducted on increasingly large populations of human subjects. The average time it takes to bring a pharmaceutical product to the market is 10.5 years, according to the Biotechnology Innovation Organization (BIO), while the Pharmaceutical Research and Manufacturers of America (PhRMA) put the average range at 10 to 15 years. In contrast, not only do supplement manufacturers get to skip this lengthy process of clinical testing, but they can introduce a supplement product to the market with as little as 75 days’ notice to the FDA.
This lengthy, rigorously regulated process doesn’t just delay the manufacturer’s receipt of profits. It also requires a huge, and often risky, investment. According to an article that appeared in the Journal of the American Medical Association (JAMA) in 2020, the median capitalized cost to bring pharmaceutical medications to the market between 2009 and 2018 was $985.3 million.
As Harvard Medical School noted in its Supplements scorecard, despite the extensive clinical testing and trials over the counter and prescription drugs undergo, “problems still occur,” and it’s not unusual for pharmaceutical medications to be recalled or required to update their labels to reflect new health warnings even after years on the market. In some cases, drug manufacturers are sued by consumers who allege that taking the medication caused them serious injuries.
Calls for Increased Regulation of Dietary Supplements
Without the authority to set approval requirements for dietary supplements—namely, regarding safety and efficacy—regulating agencies like the U.S. Food & Drug Administration are limited in their ability to protect public health. That’s why numerous parties are pushing for change.
Organizations like the Center for Responsible Nutrition have advocated for mandatory product listing with the FDA, which the association claims will improve transparency in the supplement marketplace. The Pew Charitable Trusts echoed this sentiment calling for a mandatory product listing but goes further, arguing that legislation should clarify that the Food and Drug Administration should have the ability to mandate the recall of a supplement product that is contaminated with a drug.
The debate over how rigorous regulation of the dietary supplement industry should be is complex and nuanced, as STAT News reported in 2015.
Calls for increased regulation of the dietary supplement industry aren’t new. Harvard Medical School published an article in which it argued that the “FDA needs stronger rules to ensure the safety of dietary supplements” in 2012. Regulatory concerns regarding the dietary supplement industry also aren’t limited to the United States. According to the researchers behind an article published in the journal Nutrients in 2018, regulatory agencies in other countries are also facing these concerns over the quality and safety of dietary supplements “as the marketplace for them becomes increasingly global. “
3 Ways the Supplement Industry Can Improve
Manufacturers in the supplement industry don’t have to wait until new laws are passed to start addressing safety concerns or improving the quality of their products. They can start improving their own products and, in turn, raising the bar for the industry as a whole at any time.
Here are three ways companies in the dietary supplement market could improve the industry, starting with their own products and processes.
1. Make Labels More Transparent
Perhaps one of the easiest ways to make the supplement marketplace better is for companies to adopt more transparent labeling processes. This step doesn’t even require changing supplement product formulations or product development processes, so it’s not as costly as other actions that would improve the supplement marketplace.
Transparent Labeling and Proprietary Blends
Some dietary supplement manufacturers are already doing an excellent job providing detailed information on their product labels. Often, these companies even emphasize their transparent product labels as evidence of their commitment to quality.
However, not all dietary supplements are so forthcoming in their labeling. Instead of telling consumers directly what’s in their product—and how much of each ingredient—supplement manufacturers may instead list “proprietary blends” that lack specific ingredient and dosage details.
The Prevalence of—and Problems With—Proprietary Blends
Proprietary blends are more popular in some segments of the dietary supplement industry than others. The researchers behind an article published in the journal Nutrients in 2019 reported that more than half—58%—of the pre-workout supplements they studied contained at least one proprietary blend in their formulations.
For companies that manufacture supplement products, there are many potential benefits to including proprietary blends, rather than specific ingredient and dosage information, on a product label. In most cases, though, these benefits are one-sided—and some are even detrimental to the consumer.
The most apparent reason a company might use a proprietary blend is to protect trade secrets like secret ingredients or formulas from competitors who might copy them.
According to Vibrant Health—which, as a supplement company itself, is admittedly not an unbiased source—there are a number of other reasons a company may use proprietary blends. These reasons may be disingenuous, if not downright deceptive.
In marketing messages, proprietary blends can take on more value in a consumer’s mind than their individual ingredients actually hold in real life. Calling a blend of run-of-the-mill ingredients by a catchy name can make consumers believe they’re getting something special—potentially, something worth paying more for compared to a product that includes the same ingredients.
However, tricking supplement users into thinking a blend with a fancy name is worth more than its components isn’t the only way supplement manufacturers can use proprietary blends to prey on consumers. Proprietary blends conceal the true composition of a supplement formulation.
For all a consumer knows, in a blend that includes one or two sought-after ingredients and the remaining cheaper and less valuable ingredients, the good stuff may account for just a tiny fraction of the formulation. By classifying it as a proprietary blend, the supplement manufacturer can get away with cutting corners on the quality of their formula, whereas—if the dosages were listed on the label—consumers would see for themselves that the dosages of the ingredients don’t stack up to competitor’s formulations.
This isn’t to say that all supplement products that use proprietary blends should be avoided or that their manufacturers are purposely trying to hide something. Because proprietary blends are so common in the dietary supplement industry, it’s possible that manufacturers may label their products this way just because it’s the standard in the dietary supplement market.
The Benefits of Transparent Labeling of Dietary Supplements
Changing the standard practices of dietary supplement labeling doesn’t require new laws and regulations. All it takes is for companies to be willing to be transparent about their formulations and for consumers to choose to support—through their purchases—products that don’t hide behind proprietary blends in their formulations.
Besides concerns about quality and value, proprietary blends can also make it difficult for supplement users to assess the safety of a supplement and evaluate any potential risks to their health, especially if they’re taking more than one dietary supplement. How can consumers calculate the full amount of any vitamin, mineral, botanical ingredient, or another ingredient they’re ingesting, through supplement products and the diet they consume, if the amount of the ingredient they’re taking is concealed in a proprietary blend? Although many supplement users tend to assume that these products are harmless, that’s not always the case.
Taking too high quantities of the concentrated doses of vitamins, minerals, and other ingredients found in the formulations of dietary supplements “can lead to negative health outcomes,” Healthline reported. Among these risks are the potential for gastrointestinal issues, neurological problems, vision impairment, high blood pressure, heart abnormalities, organ damage, hemorrhagic stroke, coma, and even death due to vitamin toxicity.
2. Invest in Third-Party Laboratory Testing
Adulteration of ingredients and formulations isn’t always fully the supplement manufacturer’s fault. Supply chain issues related to transparency and availability can also affect what goes into the formulation and in what amount.
Given the significant health concerns and the potential ramifications for athletes, some companies in the private sector dietary supplements market take safe manufacturing practices very seriously and are proactive about the prevention of contamination. Consumers can recognize these companies by their use of third-party laboratory testing, which supplement manufacturers often position as a selling point.

What Is Third-Party Laboratory Testing of Supplements?
Third-party testing means that an organization other than the company that made the supplement examines and tests the product in a laboratory setting to assess its contents and quality.
- The NSF (formerly the National Sanitation Foundation), a global health and safety organization that awards the NSF International Certified for Sport designation
- Labdoor, an independent for-profit company that both tests supplements purchased off of retail shelves and offers independent testing services directly to supplement companies
- ConsumerLab.com, which independently tests supplements and other consumer products through its Product Reviews and Quality Certification programs
- U.S. Pharmacopeia, an independent nonprofit organization that tests dietary supplements, medicines, and food
Because contamination with banned or otherwise potentially harmful ingredients is worryingly common in the supplement industry, the U.S. Anti-Doping Agency (USADA) encourages athletes to stick to dietary supplements that have earned certification through a third-party testing program.
Why Third-Party Testing Matters (Even If You’re Not an Athlete)
Third-party product testing gives consumers peace of mind. It’s one thing for a company to position its product as safe and effective, but it’s another thing for an independent organization or company to validate these assertions based on scientific testing.
Avoiding banned products is particularly important for athletes and others involved in formal competitions because the use of banned substances could disqualify them. However, these substances are banned for a reason, which often has to do with the health risks the illegal ingredient poses. Even if you don’t play for any sort of team and aren’t gearing up for a formal competition—if you exercise recreationally just to stay healthy and fit—it’s still important to know what you’re putting in your body.
Selecting a third-party laboratory-tested supplement is beneficial even if you aren’t at risk of being disqualified from a competition. The more companies begin embracing routine third-party testing of their products, the more options consumers will have for choosing a supplement that has been proven to contain what it says it contains.
3. Conduct More Extensive Research and Development Testing
Several of the biggest problems in the supplement industry share a common root cause: not enough testing.
Why Testing Is Minimal in the Dietary Supplements Industry
Unlike pharmaceutical drugs, which are subject to much more rigorous requirements that can take upwards of a decade to satisfy, dietary supplements encounter very few obstacles to be sold on the U.S. market. As the Food and Drug Administration explained, “FDA does not test dietary supplements before they are sold to consumers.” Since the companies that manufacture and sell dietary supplements aren’t required to undertake years of multi-stage clinical trials, they are able to bring products to the market at a low cost.
Few of these companies perform anywhere near the amount of clinical testing their counterparts in the pharmaceutical industry are required to do—even though they certainly could undertake this research—because of the high costs of investing in a series of increasingly large clinical trials.
Why Dietary Supplement Manufacturers Should Invest More in Research and Development
Let’s forget the prohibitive costs for a moment, though, and consider instead what the returns on the supplement industry’s investment in more extensive research and development could look like. How can you increase the effectiveness of supplements? The answers to this critical question lie in furthering research and development.
More clinical testing allows supplement developers to gather more evidence about the safety and efficacy of their supplement products. For many of the supplement ingredients on the market, including herbal and botanical ingredients, the biggest drawback is that the evidence of their efficacy in doing what they claim to do is limited. More research produces more evidence that can further establish the health benefits of a dietary supplement.
Instead of making potentially fraudulent claims—or simply unproven claims—companies in the dietary supplement industry can put together accurate health claims based on scientific evidence. This outcome not only protects supplement manufacturers from getting into legal trouble but also allows consumers to make more informed decisions about the supplements they’re putting in their bodies.
Through more clinical testing and trials, supplement manufacturing companies can learn more about adverse events and the potential for risks as well as health benefits. This testing can shed light not only on what health problems are reported but also on why these issues may develop. This information can help protect consumers, because the company could include additional warnings that relate to risk factors or even decide to change the formulation to avoid these safety issues altogether.
It isn’t only the safety and efficacy of existing supplement products that more focus on research and development could improve. By researching and developing different needs and products that meet them, manufacturers can make supplement products more inclusive.
For example, offering a supplement in a variety of forms—including pill, tablet, capsule, soft gel, gummy, and liquid form—allows a company to accommodate different needs, from physical disabilities that prevent a person from swallowing a pill to hectic schedules that may make mixing powder-form supplements into liquids inconvenient. Additionally, some supplements may be more effective if consumed in one form than another.
The Downsides of More Extensive Testing
A major reason supplement manufacturers aren’t voluntarily engaging in more extensive research testing of their products is the cost of undertaking a sequence of large, long-term studies on human subjects. The low barriers to entry to the U.S. market allow manufacturers to make and market their products at a relatively low cost (at least, compared to prescription drugs).
If more extensive research testing became standard in the supplement industry, whether due to companies voluntarily undertaking these studies or as a result of changes in regulations, the costs of bringing a supplement to the market would likely increase quite a bit. Those expenses would almost certainly be passed onto the consumer, and the cost of supplements would rise—potentially pricing the most necessary supplements, like vitamins, out of the budgets of those who need them the most.
Because of this downside, changes in the research, development, and testing process for dietary supplements should be made thoughtfully, with balance in mind. Just as too little regulation of the market can pose public health risks, too much regulation could keep dietary supplements from achieving their full potential in enhancing the wellness of users.
What the Supplement Industry Does Right
Although there are a lot of ways for the supplement industry to improve, there’s also a lot that the supplement industry gets right.
Combatting Malnutrition
Although many forms of dietary supplements are extras—like the sports supplements intended to help users get more results out of their workouts and beauty supplements that improve the appearance of skin, hair, and nails—nutritional supplements like vitamins do offer important potential health benefits. In many cases, dietary supplements increased users’ levels of critical nutrients that, without the supplement, they were lacking.
Not consuming a diet that meets the nutritional requirements for one’s age, developmental stage, lifestyle, and health status—whether due to poverty, access to healthy foods, food insecurity, or other reasons—is a key issue in public health. In a 2017 article published in the journal Nutrients, researchers reported that taking dietary supplements “significantly increased nutrient intakes and decreased the percent population with inadequate intakes… for most nutrients in all race/ethnic population subgroups.”
For populations for whom simply eating a healthy, balanced diet is easier said than done, having access to dietary supplements like multivitamin-mineral supplements increased consumption of critical nutrients. This outcome matters because, as the U.S. Department of Agriculture reported, chronic diseases such as cancer, cardiovascular disease, and diabetes are linked to “dietary shortfalls” that often accompany food insecurity. While taking dietary supplements isn’t a replacement for eating healthy foods, these ingredients have proven to, at least in some cases, offer benefits to otherwise malnourished individuals.
A person doesn’t have to be food-insecure to suffer from a nutritional deficiency. In fact, in a 2020 article published in Nutrients, researchers noted a “prevalence of inadequacy” that amounted to 95% of the U.S. population for vitamin D, as well as 84% of the population for vitamin E. Further, 46% of the population had a prevalence of inadequacy for vitamin C, and so did 45% of the population for vitamin A. It’s no wonder, then, that daily multivitamin supplements and vitamin D supplements are among the most popular dietary supplements in America.
It’s worth noting that a market size, share and trends analysis report by market research and consulting company Grand View Research for the dietary supplements industry identified as major drivers of market growth “changing lifestyles and hectic work schedules among working adults.” Even a person who has access to healthy foods and the financial resources to afford them may struggle to find the time to prepare, cook, and eat these foods in the midst of their busy lifestyles. Supplements make it possible to get vital nutrients even when life gets in the way of achieving an optimally healthy lifestyle.
Marketing Products for Wellness
To understand more about the success of the dietary supplement products industry, it helps to examine the question, “Why is the supplement industry growing?” In large part, this growth arises out of the industry’s highly effective efforts at marketing products for wellness and overall health.
Marketing Successes in the Dietary Supplements Industry
One look at the growth of the supplement industry proves that manufacturers in this market have achieved a lot. The global revenue for the dietary supplements market amounted to $151.9 billion in 2021, Grand View Research reported, and the global revenue amount was expected to climb to $327.4 billion by 2030.
Notably, most supplements aren’t prescribed by a doctor but rather sold over the counter, Grand View Research reported (although doctors or other health professionals may have recommended these OTC supplements. By and large, supplement consumers are taking it upon themselves to choose dietary supplements to take. They’re paying out of pocket for these wellness supplements and, often, identifying their own health needs.
Shaping Consumers’ Favorable Opinions of Dietary Supplements
Besides demonstrating impressive market growth over the past few decades, the supplement marketplace has accomplished the feat of winning over public opinion. According to a recent survey conducted by the Council for Responsible Nutrition, 77% of the U.S. population, as well as 84% of supplement users, found the supplement industry “trustworthy” in 2022.
The favorable opinion of the industry is particularly striking when compared to the overwhelmingly negative opinion of the related yet notably distinct pharmaceutical industry. A Gallup, Inc. Americans’ Views of U.S. Business and Industry Sectors poll in 2022 determined that 58% of American adults had a negative perception of the pharmaceutical industry, and only 25% had a positive opinion of the industry.
What Is the Future of the Supplement Industry?
The current issues, like contamination, potential health concerns, and regulations, could become the biggest challenges to the future of the dietary supplement industry. Finding ways to get ahead of these issues can help companies set themselves apart from less reputable supplement manufacturers and be better positioned to comply with any changes in regulations that may develop.
Additionally, taking steps like expanding their research and development processes, voluntarily undergoing third-party testing, and committing to more transparent labeling practices can help supplement manufacturing companies better take advantage of the trends that are fueling the market growth.
The future of the supplement industry is bright. Consumer awareness of the importance of optimal health and wellness isn’t going to fade away anytime soon. The overall positive perception consumers have of supplements means they are likely to continue turning to these products to help them stay well. Within the supplement industry, though, competition for sales can be fierce. The more positive steps a supplement manufacturer takes to set themselves and their products apart from the competition, the greater share of the market they are likely to command.
